Difference Between Heat and Work in Tabular Form
Contents
- Work and heat are the two most important theories in thermodynamics. Both are highly related but they are not the same. The Key Difference Between Heat and Work is that Heat is the transfer of thermal energy between systems, while work is the transfer the mechanical energy between two systems.

Comparison Chart
| Heat | Work |
|---|---|
| Heat is a form of energy. | Work is the amount of energy transferred by force through a distance. |
| Heat Requires Temperature difference. | Work Requires Force and Displacement. |
| Q > 0 when the environment is at a higher temperature than the system. Energy is transfer to the system. | W > 0 when gas is compressed. Energy is transfer into the system. |
| Q < 0 when the system is at a higher temperature than the environment. Energy is transferred out of the system. | W < 0 when a gas expands. Energy is transferred out of the system. |
| Heat is low-grade energy. | Work is high-grade energy. |
| The efficiency of the transfer of heat to work is lower. | The efficiency of the transfer of work to heat is higher. |
What’s the difference between heat and energy?
Work
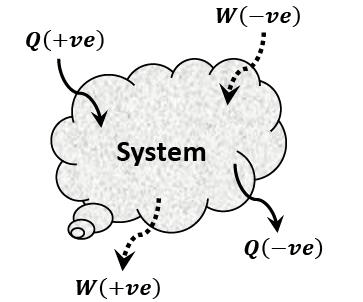
- If the work is done by the system on surrounding, e.g. when a fluid expands pushing a piston outwards, the work is said to be positive.
- Work output of the system = + W
- If the work is done on the system by surrounding e.g. when a force is
- applied to a piston to compress a fluid, the work is said to be negative.
- Work output of the system = – W
What is heat in thermodynamics?
Heat
- In general, the heat transferred to the system is considered as positive heat (+Q) while the heat transferred from the system is considered as negative heat (-Q).
Similarities
- Both are energy interactions.
- Both are transient phenomena.
- Both are boundary phenomena.
- Both represent energy crossing the boundary of the system.
- They are not the property of the system.
- Both are path functions.
Dissimilarities
- Heat transfer is the energy interaction due to temperature difference only while work is not.
- Heat is low-grade, while work is high-grade.
- Heat is thermal energy transfer, while work is mechanical energy transfer across the system boundary.
